Last Updated on June 3, 2023

The danger of suicide doubles in under the threats to adults could have already been underestimated, researchers found and 18s
Antidepressants can increase the chance of suicide, the largest review has found, as pharmaceutical companies were accused of neglecting to report side effects as well as deaths linked to the drugs.
An evaluation involving more than 18,000 people – found – of 70 trials of the very most typical antidepressants they doubled the risk of suicide and competitive behavior in under 18s.
Although a similarly blunt connection had not been seen in adults, the writers said misreporting of trial data might have caused a ‘serious underestimation of the damages.’
For years families have maintained that medical businesses and physicians who maintained a connection was unproven have drove their family members to commit suicide, but consistently blown off antidepressant drugs.

The review – the largest of its type to the impacts of the drugs – was performed from the Nordic Cochrane Centre and analysed by University College London (UCL) who today support the findings in a editorial in the British Medical Journal (BMJ).
After comparing clinical trial info to real patient reports the scientists found pharmaceutical companies had often misclassified departures and suicidal occasions in individuals taking antidepressants to “favour their products”.
Specialists said the review’s findings were “startling” and said it was “deeply worrying” that clinical trials seem to have now been misreported.
Dr Joanna Moncrieff from University College London said: “Individuals in the UK are have more than four times as several antidepressants as they did two decades past. Regardless of this, we still don’t completely comprehend the consequences of the drugs.”
The UK now has the seventh highest prescribing speed for antidepressants in the Western world, with around four million Britons taking them each year – twice as many as a decade ago in a price of more than GBP200 million a year for the 53 million prescriptions now composed.
The review looked in the five most common drugs prescribed for depression in Britain – including Prozac.
It analysed the published summary reports given to drugs regulators by pharmaceutical companies and compared it using the raw data.
Tarang Sharma of the Nordic Cochrane Centre, Copenhagen, Denmark declared: “The investigation indicates that clinical study reports, on which choices about market authorisation are based, will likely underestimate the degree of drug related damages.”
One unnamed pharmaceutical company, who asserted they’d happened following the trials had ceased misreported four departures.
One patient strangled himself out of the blue but he was excluded in the results because it had been asserted he was about the trial while he was dying in hospital because he lived for five days.
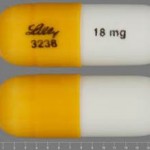
Over half of suicidal ideas and the suicide attempts had been misrecorded as worsening of depression or mental instability. In outline trial reports in the drugs giant Eli Lilly, suicidal efforts missed in 90 per cent of instances.
One dad whose son committed suicide has set up the anonymous campaigning web site AntiDepAware and continues to be tracking suicides associated with antidepressant drugs for the past three years.
So there have been at least 35 inquests with departures linked to antidepressants. There were more than 450 last year.
“I can say, hand on heart, that I do not recall reading a report of an inquest where a suicide verdict was applied to a kid who’d never been on any psychiatric medication,” he said.
Professor Peter Goetzsche, the lead writer in the Nordic Cochrane Centre said: “Antidepressants do not work in kids, that’s quite clear, in the randomised trials kids say that they do not work for them, but they raise their risk of suicide.
“What I get out of the colossal coverage of suicides is the fact that SSRIs likely increase suicides in most ages.”
The writers reasoned that psychotherapy and that exercise needs to be offered before antidepressants because that damages are prone to outweigh the advantages to kids and young adults.
The drugs which reviewed were Duloxetine, Fluoxetine – that’s also referred to as Prozac – Paroxetine, Sertraline and Venlafaxine which belong to two groups, Selective serotonin reuptake inhibitors antidepressants (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs).
Commenting on the findings of the review Professor Gotzsche included, “It’s completely terrible they have such disregard for human lives.”
Lucie Russell, Director of Campaigns for YoungMinds: “This new study is greatly worrying, and it is quite concerning that some clinical trials are misreported or badly designed.
“Kids as well as their parents will need to have solid and complete information regarding the results that antidepressants may have to allow them to make educated choices about treatment.
“We consider that prescribing antidepressants should not ever be the sole plan of action.”
Marjorie Wallace, Chief Executive of the mental health charity SANE included: “This new report suggests that great caution should be taken in prescribing new-generation antidepressants (SSRIs) for youngsters and teens.”
Health specialists said misreporting of trial data proved to be an issue that was growing.
Dr Paul Ramchandani, Consultant Child and Adolescent Psychiatrist with Central North West London Foundation NHS Trust said: “There happen to be issues to get several years in regards to the manner in which some trials of medications or drugs for depression were initially reported, especially when the treatment was for youngsters and teens.”
Dr Paul Keedwell, Consultant Psychiatrist and Senior Research Fellow in the Neurobiology of Mood Disorders at Cardiff University, included:”The findings give a further lesson in how professionals must carefully scrutinise drug company overviews for data on adverse events.”
Yet drug companies maintained their data, saying that security was their top priority.
A spokesman for Eli Lilly said: “No regulatory authority has ever discovered that Lilly withheld or improperly divulged any data related to these drugs.
“Put simply, our aim will be to make life better for individuals all over the world, and Lilly is dedicated to sharing the outcomes of our clinical trials and ensuring this info can be obtained to the people that desire it.”
CASE STUDY
Dr Margaret Tisdale, was a distinguished virologist who helped develop treatments for AIDS, HIV and flu.
The church warden was a well known pillar of Wrestlingworth of the community in her hamlet in Bedfordshire, as well as a chair of school governors in the area primary.
But last year she was discovered unconscious in her garden having fallen in the window. Was a copy of a self-help book on coping with her antidepressant pills and anxiety.
An inquest October that was last heard in the months resulting in her departure she’d stressed in regards to the level of a physician prescribed Citalopram and voluntary work she’d taken on.
A coroner ruled that she’d committed suicide after being ‘overtaken by anxiety.’
Yet in the hearing, issues were raised by her sister Linda Foreman in regards to the negative effects of her drugs.
She said: “I believed that she was not depressed, but was instead quite nervous and stressed. After I looked up the unwanted effects I was concerned regarding the Citalopram she was prescribed. I do not believe she understood how serious the side effects could be.”
Speaking following the hearing she included: “Margaret wasn’t depressed but suffering from tension and tension.
“I now realize that antidepressants are prescribed too often and much better results could be attained through counselling.”
Taking antidepressants
Almost one third of those who attempt to avoid taking antidepressants experience withdrawal symptoms, based on an 2014 survey from the Royal College of Psychiatrists. The survey of more than 800 individuals discovered that nervousness, dizziness, “head zaps”, stomach upsets, influenza-like symptoms and depression were among the most frequent aftereffects of withdrawal. One quarter of these in the survey were not aware there could be difficulties linked to preventing antidepressants. The Faculty advises that anyone who wants to avoid taking antidepressant drugs should:
- Talk about the options with their physician and be alert to potential withdrawal
- Pick a time that is good
- Seek support from family and friends and inquire whether you are going to be needing time off work
- Decrease the dose
- Prepare yourself if desired to raise the dose
- Maintain a diary of your symptoms and drug doses
- Keep a watch in your disposition, look after yourself and keep active
- Practice cognitive behavioural treatment (CBT) and relaxation techniques
- If you’re concerned about the way you feel, return to your own physician
The Causes Of Antidepressant Discontinuation Syndrome?
There is really no method to call in the event that you’ll have discontinuation symptoms after discontinuing an antidepressant. Scientists aren’t exactly certain why antidepressant discontinuation syndrome is developed by many people while others don’t.
Antidepressants help restore the standard function of naturally occurring, mood-modulating materials in the mind, called neurotransmitters, including norepinephrine and serotonin. Some mental health experts theorize that suddenly stopping an antidepressant just will not give your brain time to adapt to the changes that are fast.
Should antidepressants be discontinued?
You need to always speak with prescriber your GP or pharmacist in case you are considering quitting your antidepressants.
A dose of antidepressants needs to be reduced over a couple of weeks over 6 to 8 weeks, and if treatment has lasted less than eight weeks if treatment has continued six to eight months.
The reason being although antidepressants will not be classed as medications that are addictive, they are able to cause serious withdrawal symptoms if discontinued suddenly. These symptoms could be similar or completely new to a few of the first symptoms of the sickness.
Withdrawal symptoms
Withdrawal symptoms are determined by the kind of antidepressant. For example:
selective serotonin reuptake inhibitors (SSRIs), tricyclic antidepressants (TCAs) and Venlafaxine usually result in a flu-like illness (chills, muscle pain, excessive perspiration, nausea and headache) and sleeplessness
Venlafaxine and sSRIs may also cause dizziness or electric shock sensations
monoamine oxidase inhibitors (MAOIs) can cause symptoms like irritability, agitation, sleep difficulties and motion disorders
The start of withdrawal symptoms is can normally continue for up to six weeks and normally within five days of discontinuing the medication. If symptoms are severe, alternative strategies may need to be used, for example diminishing the dose slowly and reintroducing another antidepressant from the exact same group, or quitting fully and handling your symptoms.
Keep in touch with prescriber or your GP to agree the most effective strategy for you personally.
The Royal College of Psychiatrists has proposed several things that you are able to do before, during and once you have quit taking antidepressants that will help you in getting through the procedure.
Taking antidepressants
Remain connected together with specialist nurse or your GP when you begin taking antidepressants. You need to continue taking the antidepressants for at least four weeks (six weeks in case you are elderly) to see how well they have been working.
Treatment needs to be continued in the exact same dose for at least six months into a year, in case your antidepressants are working. You need to continue to receive care treatment for as many as five years, or maybe even more, for those who have a continued history of depression.
For those who have some issues relating to this information, or want any help understanding it and connecting it for your personal scenario, you need to speak with your GP or pharmacist (chemist). It is possible to also telephone NHS 111.